- What are Hydrogen and ammonia?
- Hydrogen and ammonia business value chain
- Current status and outlook of Hydrogen and ammonia
What are Hydrogen and ammonia?
What is hydrogen?
Hydrogen (H2) is a colorless, odorless gas at room temperature, with no taste or smell. It is the lightest of gases and a combustible gas. Unlike other combustible gases, its flame is colorless and transparent. When it burns, it reacts with oxygen to become water, emitting no CO2.
Although hydrogen exists abundantly on Earth in compounds with other elements such as water, it is scarce as a usable element on its own. In recent years, there are reports of hydrogen being naturally produced in the ground or on the seafloor and existing as a standalone element.
Does hydrogen have a color?
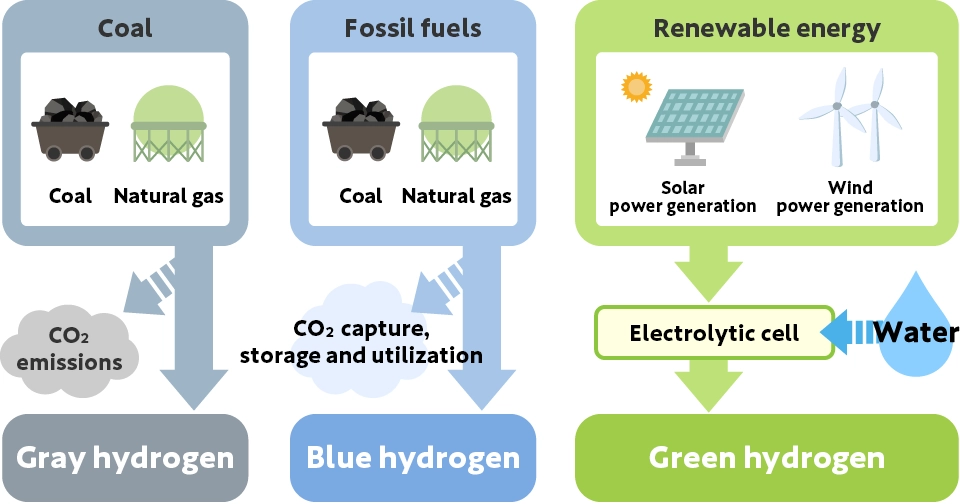
Hydrogen is colorless, but depending on the production method, it is categorized and referred to by different colors (albeit without a universally agreed definition). The main color categorization of hydrogen is as follows:
-
Gray hydrogen: Hydrogen produced using coal or natural gas as raw materials
-
Blue hydrogen: Hydrogen produced using natural gas as a raw material, where carbon dioxide (CO2) emitted during production is reduced by carbon capture and storage (CCS*) technologies
-
Green hydrogen: Carbon-free hydrogen produced by electrolyzing water using renewable energy
(Source) Agency for Natural Resources and Energy
What is ammonia?
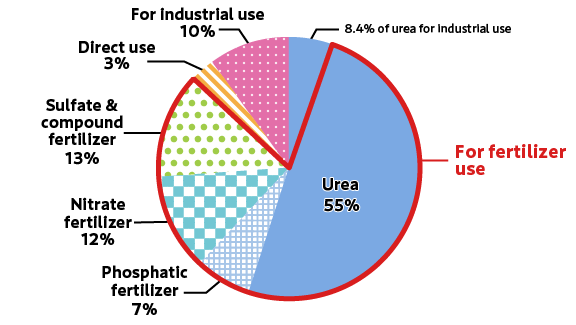
Ammonia (NH3) is a colorless, transparent gas at room temperature and atmospheric pressure, composed of hydrogen (H) and nitrogen (N). It has a unique, strong, pungent smell and is mainly used as a raw material for fertilizers. In addition, it is used as a raw material in a wide range of applications such as chemical products like melamine resin and synthetic fibers, stain removal from clothes, etc. About 200 million tons of ammonia are produced annually worldwide.
Ammonia can easily be liquefied by pressurization and cooling. Also, when released into the air, it turns into white smoke.
(Source) The Institute of Energy Economics, Japan and NEXANT (2012)
Features of Hydrogen and ammonia
Safety of Hydrogen and ammonia
While Hydrogen can be perceived as being prone to ignition or explosion, it can be safely used if handled correctly. Following the precautions in handling hydrogen, such as "don't leak it", "detect and stop any leaks immediately if they occur", and "properly ventilate, don't trap leaked hydrogen", makes Hydrogen a safe energy source when used correctly.
On the other hand, Ammonia is classified as a 'hazardous substance', but technologies to safely use it have been established. Both substances, when used and handled carefully following established safety measures and protocols, can be safe for industrial and potential energy use.
Why are Hydrogen and ammonia in the spotlight now?
Hydrogen and ammonia are garnering attention as clean energy sources that do not emit CO2 or harmful substances when burned, hence they are environmental-friendly. They are also expected to play a vital role in decarbonizing sectors where electrification is a challenge, such as the steel and chemical industries, and are being researched for use in fuel cells.
Hydrogen, which can be produced not only from fossil fuels but also from water using natural energy like solar and wind power, is seen as a potential means to improve energy security for resource-poor countries like Japan. Although hydrogen itself is a clean energy that does not emit CO2 when used, CO2 is emitted if natural gas is used in its production, as well as if fossil fuels are used to generate the electricity needed for electrolysis. The challenge lies in how to reduce the CO2 emitted during the production of hydrogen. Efforts are underway to sequester the CO2 emitted during hydrogen production through carbon capture, utilization, and storage (CCUS) or to perform electrolysis using electricity generated from renewable energy sources.
Ammonia can also be utilized as a "carrier" of hydrogen and can also be used as a fuel directly without being converted into hydrogen. Given ammonia’s worldwide application track record and established safety measures, existing infrastructure and supply chains can be utilized, and it can be produced and utilized at lower costs compared to hydrogen. Research is being conducted on strategies that involve converting large volumes of hydrogen into ammonia for transportation, and then converting it back into hydrogen at the point of use.
Hydrogen and ammonia business value chain
Hydrogen and ammonia can be produced using fossil fuels or electricity from renewable energy sources. Hydrogen can be stored by compression or liquefaction, or it can be converted into other substances, such as ammonia, for transportation. Demand is expected to grow in the future in fields such as electricity generation, steelmaking, and mobility.
Since ammonia can be burned directly as fuel, technological development is underway to generate electricity solely from ammonia combustion in the future. There are also ongoing demonstration experiments to reduce CO2 emissions by mixing ammonia in coal for thermal power generation (co-firing).
These strategies are part of a larger plan to reduce carbon emissions and shift towards renewable energy sources. They also represent the potential for a new energy infrastructure that could reshape the way we generate, store and use energy.
Hydrogen and ammonia business value chain
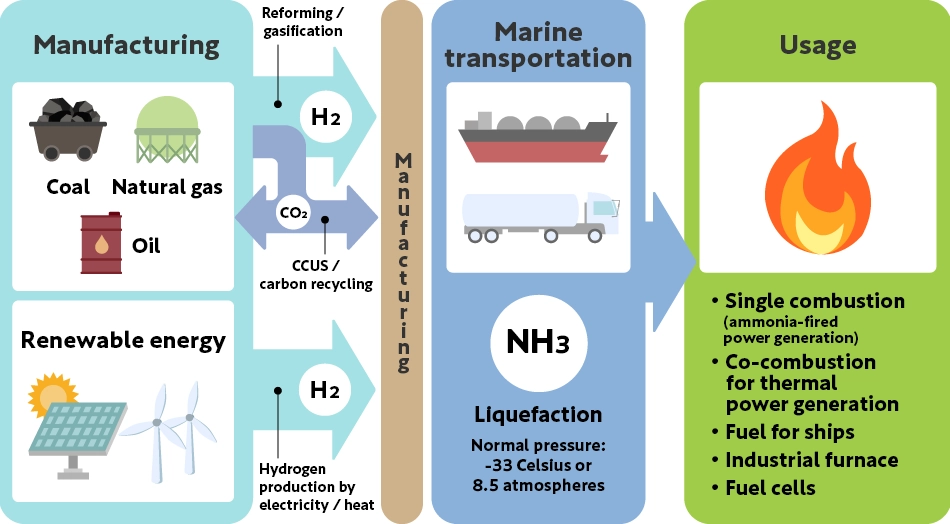
Future expanded usage of hydrogen
| Use Case | Usage Method | Challenges |
|---|---|---|
| Electricity |
|
|
| Mobility |
|
|
| Steelmaking |
|
|
Current Status and Outlook of Hydrogen and ammonia
In the hydrogen manufacturing business, especially in the case of blue hydrogen, we can use our existing natural gas assets, and leverage our technological strengths accumulated in upstream operations (subsurface/drilling), LNG operations and transportation (marine and pipeline). However, there are technical and economic challenges, so we must cooperate with other companies and build an environment where hydrogen can be widely used as fuel.
We are also challenging advanced initiatives. We are constructing a plant in Kashiwazaki City, Niigata Prefecture, for the demonstration of blue hydrogen production and usage, which will reduce the CO2 emitted when producing hydrogen from natural gas by using CCS (carbon capture and storage). Also, we are advancing a joint project with overseas companies aiming for the commercial production of low-carbon ammonia in Texas, USA. We will continue to work on promoting Hydrogen and ammonia, such as shifting consumer focus towards energy conversion by utilizing existing petroleum and natural gas supply routes and value chains.